If you have received either the Moderna or the Pfizer/BioNTech vaccine against COVID-19, you benefiting from a biomedical tool called CRISPR. CRISPR technology enabled scientists to create both vaccines in record time, bypassing the clumsy and time-consuming methods employed in vaccine development in the past. The historic breakthrough that led to the now-widespread use of CRISPR in biomedical labs the world over came only in 2012. And it won the Nobel Prize for Chemistry eight years later for a remarkable woman at the University of California, Berkeley, named Jennifer Doudna.
Four main characters
Doudna’s work, and that of her students, post-docs, and colleagues as well as collaborators and rivals from other labs are the subject of Walter Isaacson’s gripping account in The Code Breaker. The publisher markets the book as a biography, and Doudna is the central subject. But there are “four main characters: Jennifer Doudna, Emmanuelle Charpentier, George Church, and Feng Zhang.” And they share the spotlight with dozens of other scientists as well as a handful of iconic figures in the biomedical establishment, including Francis Collins (National Institutes of Health), David Baltimore (California Institute of Technology), and James Watson (co-discoverer of DNA’s double helix). Much more than a biography, this is the story of CRISPR and its potential to upend the way medicine is practiced in the twenty-first century.
The Code Breaker: Jennifer Doudna, Gene Editing, and the Future of the Human Race by Walter Isaacson (2021) 552 pages ★★★★★
CRISPR technology explained
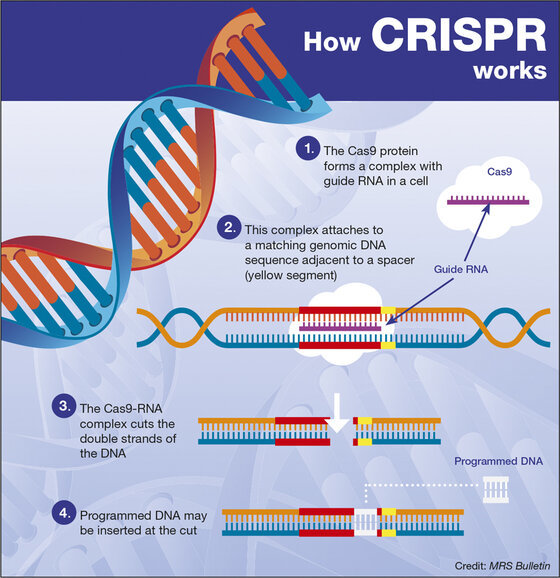
“The gene-editing tool that Doudna and others developed in 2012,” Isaacson notes, “is based on a virus-fighting trick used by bacteria, which have been battling viruses for more than a billion years.” Bacteria produce proteins to combat attackers. These proteins are catalytic chemicals called enzymes that target a virus’ genetic code and carve out tiny snippets of its RNA or DNA. By inserting those snippets into its own genome, the bacteria can recognize and defeat a similar virus that attacks in the future. Doudna, Zhang, and their collaborators use some of those chemicals (known as CRISPR-associated enzymes, or Cas) to battle disease in humans. One specific enzyme, Cas9, turns out to be the Swiss Army Knife of the process. And it’s simple to use this technique. With the proper tools, all easy to obtain online, students in college labs or biohackers in their basements can master it without difficulty.
Five overarching themes
Five themes dominate Isaacson’s account of CRISPR technology: the biological versus the digital revolution, collaboration, competition, the global scope of Big Science, and the challenges of bioethics.
The genetic code vs. computer code
“The first half of the twentieth century,” Isaacson writes, “featured a revolution driven by physics. . . The second half . . . was an information-technology era . . . Now we have entered a third and even more momentous era, a life-science revolution.” The human race now possesses the ability to change the course of evolution.
“Biology has become the new tech,” Isaacson asserts. “Young innovators are buzzing about genetic code rather than computer code. The atmosphere is . . . reminiscent of when Bill Gates and Steve Jobs frequented the early personal computer shows, except this time the rock stars are Jennifer Doudna and Feng Zhang.” Isaacson adds, “people of my generation became fascinated by personal computers and the web. We made sure our kids learned how to code. Now we will have to make sure they understand the code of life.”
In the future, historians will determine “whether the digital revolution or the life-science revolution will end up being the most important.” The jury is out.
Collaboration and teamwork
In science today, little is accomplished by lone investigators working in isolation. The questions scientists face are simply too big, too complex, and frequently too expensive for all but major institutions. Science in the twenty-first century involves “an iterative dance among basic scientists, practical inventors, and business leaders.” Sometimes philanthropic foundations or venture capitalists and government institutions such as DARPA or China’s equivalent must become involved as well. In The Code Breaker, Isaacson highlights three of those institutions: UC Berkeley’s Department of Molecular & Cell Biology, the Broad Institute at MIT and Harvard, and the Cold Spring Harbor Laboratory.
Teamwork within a lab and collaboration with others elsewhere are almost always essential. Doudna (born 1964) did not win the 2020 Nobel Prize alone for the development of CRISPR technology. She shared it with her French collaborator, Emmanuelle Charpentier (born 1968), who was working at the time in Sweden. Both led laboratory work that involved teams of students and post-docs. “The effort to develop CRISPR . . . involved microbe-hunters working with geneticists, structural biologists, biochemists, and computer geeks.” But their breakthrough also came in part because of work conducted at labs elsewhere in Europe and the United States. Future collaborators they met and insights they gained at face-to-face meetings and conferences played a role as well. And they shared other major awards with some of those scientists, with whom they were in a high-pressure race to be the first to announce their results.
Competition
The history of science is rife with examples of intense and sometimes bitter competition that led to some of the biggest breakthroughs in humanity’s understanding of the world around us. The quarrel between Isaac Newton and Gottfried Wilhelm Leibniz over the discovery of the calculus. Charles Darwin’s rush to publish The Origin of Species because his younger colleague, Alfred Russel Wallace, seemed likely to scoop him. James Watson and Francis Crick‘s competition with Linus Pauling to unravel the secrets of DNA. And similar competition played itself out in the early months of 2012 in the multi-polar race to harness the power of CRISPR technology.
Dueling scientific papers
“To what extent do discoveries depend on individual genius, and to what extent has teamwork become more critical?” Isaacson asks in his introduction. “Has the competition for prizes and patents undermined collaboration?” In the pages that follow, he seems to suggest that the intense competition that characterized the rush to understand CRISPR did, in fact, jeopardize not just good feeling but the potential for productive cooperation between the labs led by Doudna and Zhang, the two principal competitors. The competition devolved into dueling scientific papers that minimized each other’s accomplishments and an “epic patent battle” between them that is “still dragging along.”
COVID-19 changed everything
But the ill feeling fell by the wayside when the time came early in the COVID-19 pandemic as the two superstars and scores of other research scientists rushed to share insights as they learned to apply CRISPR technology to the SARS-CoV-2 virus. And that collaboration helped scientists at both labs develop potential treatments for the coronavirus as well as remarkable diagnostic tools that show promise of revolutionizing the practice of medicine in years to come.
The global scope of science
Throughout the pages of The Code Breaker, despite the primary focus on Doudna and her colleagues in Berkeley, and the secondary emphasis on Zhang, the action shifts around the world as other players enter the scene. Scientists in France, Lithuania, Germany, England, Spain, Japan, the Netherlands, and China as well as the United States all appear in the story. And two researchers working on yogurt for a Danish food ingredient company achieved an important breakthrough, too; one of them even shared major awards with Doudna and Zhang. He is now the Editor-in-Chief of The CRISPR Journal.
Ethical questions emerge
Isaacson explores in depth the ethical questions raised by the new ability afforded by CRISPR technology to alter the human genome. As he makes clear, there are two ways in which this can be accomplished. In one, somatic editing, a scientist targets a single gene such as the one that leads to sickle-cell anemia. If successful, the intervention will save patients from the intense pain and early death of the disease but have no impact on their descendants.
Germline editing
Germline editing does, however. Scientists alter the human genome by adding or removing genes, and the patients’ descendants will all experience the benefit (or harm) that results. And the changes are irreversible. Isaacson explores a number of thought experiments that illustrate just how fraught with danger an intervention of this sort might be. “How do we distinguish between traits that are true disabilities,” Isaacson asks, “and ones that are disabilities mainly because society is not good at adapting for them?” For example, many deaf persons insist their inability to hear is a gift, not a handicap.
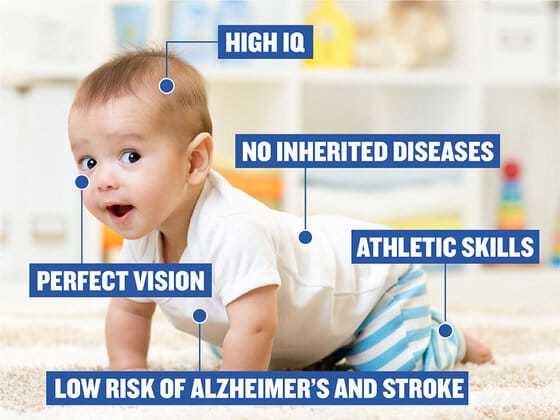
Of course, using CRISPR to eliminate a gene or genes that code for some dread disease poses fewer ethical questions. But what about altering the genome in the test tube to enhance the intelligence or the physical strength of the baby that will be born? What about coding for blond hair and blue eyes or an extra eight inches in height? Assuming it’s possible to do these things without dreadful side effects, is it right?
The world’s first designer human babies
Isaacson traces the troubled story of He Jianqui, the US-educated Chinese scientist who created the first genetically edited human babies in 2018. He made twins Lulu and Nana resistant to HIV. But he may also have caused other, unintentional changes in the process because of flaws in the procedures he followed. After a world outcry, Isaacson notes, “He was sentenced to three years in prison, fined $430,000, and banned for life from working in reproductive science.”
Peering into the future
Supersoldiers. Designer babies. Even the loss of diversity in our species, as parents widely adopt “enhancements” made possible by gene editing. And it seems to be a given that if scientists make something possible, others will find ways to profit from it. “Figuring out if and when to edit our genes will be one of the most consequential questions of the twenty-first century,” Isaacson asserts. “We have to face the potential conflict between what is desired by the individual versus what is good for human civilization.” But the world’s two hundred nations have never been able to agree on much of anything. Is it likely a consensus will emerge on how to regulate gene editing?
Democratizing medicine
The dark side of CRISPR technology’s potential notwithstanding, a great deal more good can come of this remarkable tool. “Once the delivery mechanisms are worked out,” Isaacson notes, “CRISPR-based systems [developed by Doudna and Zhang’s labs] will be able to treat and protect people without having to activate the body’s own immune system, which can be quirky and delicate.” And other tools they’ve pioneered will “democratize and decentralize medicine. The most important next steps will be innovations in ‘microfluidics,’ which involves channeling tiny amounts of liquid in a device, and then connecting the information to our cell phones. That will allow us all, in the privacy of our homes, to test our saliva and blood for hundreds of medical indicators, monitor our health conditions on our phones, and share the data with doctors and researchers.”
Would breakthroughs of that magnitude justify the downside posed by CRISPR’s ethically challenged outcomes? I’m on Jennifer Doudna’s side. But you be the judge.
About the author
Walter Isaacson (born 1952) is best known as the author of bestselling biographies of Albert Einstein, Leonardo da Vinci, Steve Jobs, Benjamin Franklin, and others. He is sometimes thought of in the same breath as eminent biographers such as David McCullough, Robert A. Caro, and Doris Kearns Goodwin. But writing appears to be a sideline for this amazing man. He is currently Leonard Lauder Professor of American History and Values at Tulane University and lives in New Orleans. In the past, he served as President and CEO of the Aspen Institute, chair and CEO of CNN, chair of the Broadcasting Board of Governors, and the editor of Time as well as other appointive posts inside and out of government.
For related reading
I’ve also reviewed two other biographies by Walter Isaacson:
- Elon Musk (A revealing new biography of Elon Musk)
- Steve Jobs (The Walter Isaacson biography of Steve Jobs)
I’ve reviewed two other books about biotech:
- Bad Blood: Secrets and Lies in a Silicon Valley Startup by John Carreyrou (A cautionary tale about corporate power in Silicon Valley)—the story of Elizabeth Holmes and Theranos
- For Blood and Money: Billionaires, Biotech, and the Quest for a Blockbuster Drug by Nathan Vardi (The drama behind two biotech startups)
This is one of The best books of 2021.
The popular website Berkeleyside has featured three stories about Jennifer Doudna and this book:
- “In new biography of Nobel Prize winner Jennifer Doudna, science is a ‘competitive sport’” by Michael Berry
- Jennifer Doudna & Lance Knobel: The CRISPR Way To Fix Genes
- “UC Berkeley lab pivots from editing DNA to processing COVID-19 tests” by Natalie Orenstein
I’ve also reviewed Steve Jobs by Walter Isaacson.
You might also be interested in 10 great biographies or Science explained in 10 excellent popular books.
And you can always find my most popular reviews, and the most recent ones, on the Home Page.


































Hi. Nice Article. Keep sharing more information related to CRISPR Technology !!